Abstract
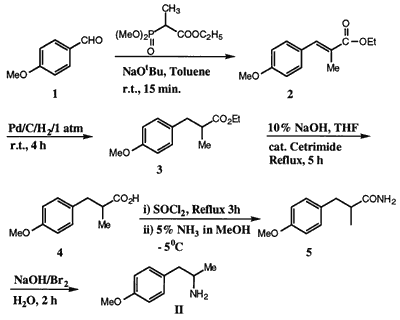
Synthesis of 2-amino-1-(4-methoxyphenyl)-propane (II) starting from p-anisaldehyde (1) in 67% overall yield is described. Key reactions involved Horner�Wadsworth�Emmons olefination of 1 to form the ester 2 and Hoffmann degradation of amide 5 to obtain the amine II.
Introduction
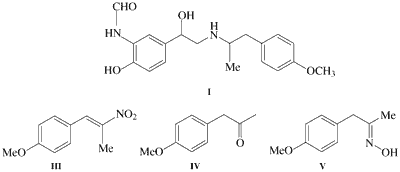
β-Arylethylamine functionality is essential to many amphetamine drugs that offer high selectivity for 2-adrenoceptors1. A variety of 2-adrenoceptor agonists have been prepared for treatment of asthma and chronic bronchitis2a-d. Among them formoterol (I) offers high selectivity for 2-adrenoceptors and has excellent safety, tolerance profile and has been commercialized in racemic form3a-d. Synthesis of I requires preparation of 2-amino-1-(4-methoxyphenyl)-propane (II) by a process which is amenable to scale up, avoids use of hazardous reagents, elevated temperature and pressure. Synthetic procedures to prepare β-phenyl ethylamine II describes Henry reaction of p-anisaldehyde (1) to obtain the key intermediate β-nitrostyrene4 III followed by hydrogenation at high pressure in presence of Pd/C5 or Red-Al4b or by refluxing with LiAlH4 in Et2O4f to obtain the amine II in 70�85% yield in one approach and in the second III was converted to the keto compound IV by reaction with Fe/aq. HCl4a, or NaH2PO2·H2O/Raney�Ni at pH 56 or HClO47 or Zn�TFA8. The keto compound IV was converted to the corresponding oxime V9b by reaction with NH2OH·HCl followed by reduction with Raney�Ni at high pressure4a to obtain the amine II in 40�43% overall yield.
We report herein a simple laboratory method for the preparation of amine II.
Horner�Wadsworth�Emmons reactionThe Horner�Wadsworth�Emmons reaction of 1 with ethyl (2-dimethoxyphosphinyl)-2- propanoate10 (1.1 mol equiv.) and NaOtBu (1.5 mol equiv.) in toluene at room temperature for 15 min gave the α,β-unsaturated ester 2 which on catalytic hydrogenation (1 atm.) with 10% Pd/C in MeOH for 4 h gave the saturated ester 3, while progress of the reaction was monitored by HPLC (experimental). Hydrolysis of 3 with aq. NaOH (20%) and a catalytic amount of cetrimide at reflux temperature for 5 h gave the acid derivative 4. Compound 4 was transformed to the amide 5 (mp: 119�121�C) by reaction with SOCl2 followed by quenching with methanolic ammonia solution at -5�C. Hoffman degradation of amide 5 by reaction with NaOBr at 70�C for 2 h gave the amine II in 67% overall yield from p-anisaldehyde (1).
Experimental
Ethyl-3-(4-methoxyphenyl)-2-methyl-2-propenoate (2)
To a solution of p-anisaldehyde 1 (32 g, 0.24 mol), toluene (160 mL) and ethyl-(2-dimethoxyphosphinyl)-2-propanoate (58.8 g, 0.28 mol) was added NaOtBu (33.6 g, 0.35 mol) at 0�C under N2 atmosphere during half an hour. The reaction mixture was brought to room temperature and stirred for 15 min. Progress of the reaction was monitored by TLC [AcOEt:Hexane; 1:4] from the appearance of a faster moving spot. After completion of the reaction, the reaction mixture was neutralised with 10% aq. HCl. The toluene layer was separated, washed with water (2�100 mL), dried (Na2SO4) and concentrated to obtain the title compound 2 as a syrup in quantitative yield (51.6 g).
Ethyl-3-(4-methoxyphenyl)-2-methyl-propanoate (3)
To a solution of 2 (32 g, 0.145 mol) in MeOH (160 mL) was added Pd/C 10% (1.0 g) and subjected to hydrogenation (1 atm) for 4 h. Progress of the reaction was monitored by HPLC (Eluant: 70% CH3CN in H2O, 2 had a retention time of 8.3 min and compound 3 of 7.3 min). After completion of the reaction, the catalyst was filtered off and the filtrate was concentrated to obtain the title compound 3 as a syrup in quantitative yield (31.8 g).
3-(4-Methoxyphenyl)-propanoic Acid (4)
To a solution of 3 (25 g, 0.11 mmol) in THF (93 mL) was added NaOH (8.96 g in 46 mL H2O) (0.22 mol), cetrimide (0.2g) and refluxed for 5 h. The progress of the reaction was monitored by TLC [AcOEt:Hexane; 2:3] from the appearance of a slower moving spot. After completion of the reaction, THF was removed on a rotary evaporator and the reaction mixture was acidified with 10% aq. HCl and extracted into CH2Cl2 (125 mL). The organic layer was separated, washed with water (2�100 mL), dried (Na2SO4) and concentrated to obtain the title compound 4 as a syrup in quantitative yield (21.5 g).
3-(4-Methoxyphenyl)-2-methyl-propanamide (5)
A solution of 4 (20 g, 0.10 mol) in SOCl2 (14.6 mL, 0.20 mol) was heated to reflux for 2 h. After completion of the reaction, excess thionyl chloride was distilled off to obtain a residue which was dissolved in MeOH (60 mL), cooled to -5�C under nitrogen atmosphere and a 5% solution of ammonia in MeOH (3.50 g in 70 mL MeOH, 0.20 mol) was added dropwise under efficient stirring at -5�C. After completion of the reaction, methanol was removed on a rotary evaporator to obtain a solid which was filtered, washed with water (100 mL) and dried to obtain the title compound 5 in quantitative yield (19.30 g); mp 119�121�C.
2-Amino-1-(4-methoxyphenyl)-propane (II)
To a stirred solution of sodium hypobromite [Br2 (4.6 mL, 0.09 mol), NaOH (18.72 g, 0.48 mol) in 150 mL water] was added a finely divided amide 5 (15 g, 0.078 mol) at 0�C during 10 min. The reaction mixture was heated to 70�C for 2 h. The progress of the reaction was monitored by TLC (BuOH:EtOH:NH3, 7:2.6:0.3 mL). After completion of the reaction, the reaction mixture was extracted into CH2Cl2 (75 mL). Organic layer was separated, washed with water (2�75 mL), dried (Na2SO4) and concentrated to obtain the title compound II as a syrup (9.6 g, 75%) that exhibited comparable 1H-NMR spectrum with that reported in literature4f,11a. For the purpose of characterisation II was also converted to the corresponding hydrochloride11b [10.5g, 65%, mp 208�210�C (lit.4a 208�C)] by treating with 5% HCl in MeOH.